Analysis Of Water
Analysis Of Water
Water
chemistry analyses are carried out to identify and quantify the chemical
components and properties of water samples. The type and sensitivity of the
analysis depends on the purpose of the analysis and the anticipated use of the
water. Chemical water analysis is carried out on water used in industrial
processes, on waste-water stream, on rivers and stream, on rainfall and on the
sea. In all cases the results of the analysis provides information that can be
used to make decisions or to provide re-assurance that conditions are as
expected. The analytical parameters selected are chosen to be appropriate for
the decision making process or to establish acceptable normality. Water
chemistry analysis is often the groundwork of studies of water quality, pollution,
hydrology and geothermal waters. Analytical methods routinely used can detect
and measure all the natural elements and their inorganic compounds and a very
wide range of organic chemical species using methods such as gas chromatography
and mass spectrometry. In water treatment plants producing drinking water and
in some industrial processes using products with distinctive taste and odours,
specialised organoleptic methods may be used to detect smells at very low
concentrations.
In
many cases the parameters will reflect the national and local water quality
standards determined by law or other regulations. Typical parameters for
ensuring that unpolluted surface waters remain within acceptable chemical
standards include pH, major cations and anions including ammonia, nitrate,
nitrite, phosphate, conductivity, phenol, chemical oxygen demand (COD) and
biochemical oxygen demand (BOD).
1.Reverse Osmosis(RO)

Reverse
osmosis (RO) is a water purification process that uses a partially permeable
membrane to remove ions, unwanted molecules and larger particles from drinking
water. In reverse osmosis, an applied pressure is used to overcome osmotic
pressure, a colligative property, that is driven by chemical potential
differences of the solvent, a thermodynamic parameter. Reverse osmosis can
remove many types of dissolved and suspended chemical species as well as
biological ones (principally bacteria) from water, and is used in both
industrial processes and the production of potable water. The result is that
the solute is retained on the pressurized side of the membrane and the pure
solvent is allowed to pass to the other side. To be "selective", this
membrane should not allow large molecules or ions through the pores (holes),
but should allow smaller components of the solution (such as solvent molecules,
i.e., water, H2O) to pass freely.
In
the normal osmosis process, the solvent naturally moves from an area of low
solute concentration (high water potential), through a membrane, to an area of
high solute concentration (low water potential). The driving force for the
movement of the solvent is the reduction in the free energy of the system when
the difference in solvent concentration on either side of a membrane is
reduced, generating osmotic pressure due to the solvent moving into the more
concentrated solution. Applying an external pressure to reverse the natural
flow of pure solvent, thus, is reverse osmosis. The process is similar to other
membrane technology applications.
Reverse
osmosis differs from filtration in that the mechanism of fluid flow is by
osmosis across a membrane. The predominant removal mechanism in membrane
filtration is straining, or size exclusion, where the pores are 0.01
micrometers or larger, so the process can theoretically achieve perfect efficiency
regardless of parameters such as the solution's pressure and concentration.
Reverse osmosis instead involves solvent diffusion across a membrane that is
either nonporous or uses nanofiltration with pores 0.001 micrometers in size.
The predominant removal mechanism is from differences in solubility or
diffusivity, and the process is dependent on pressure, solute concentration,
and other conditions. Reverse osmosis is most commonly known for its use in
drinking water purification from seawater, removing the salt and other effluent
materials from the water molecules.
2.Tap Water

Tap
water (running water, city water, town water, municipal water, sink water,
etc.) is water supplied to a tap (valve). Its uses include drinking, washing,
cooking, and the flushing of toilets. Indoor tap water is distributed through
"indoor plumbing", which has existed since antiquity but was
available to very few people until the second half of the 19th century when it
began to spread in popularity in what are now developed countries. Tap water
became common in many regions during the 20th century, and is now lacking
mainly among people in poverty, especially in developing countries.

Tap water is often culturally assumed to be drinking water, especially in developed countries. Usually it is potable, although water quality problems are not rare. Household water purification methods such as water filters, boiling, or distillation can be used when tap water's potability is doubted. The application of technologies (such as water treatment plants) involved in providing clean water to homes, businesses, and public buildings is a major subfield of sanitary engineering. Calling a water supply "tap water" distinguishes it from the other main types of fresh water which may be available; these include water from rainwater-collecting cisterns, water from village pumps or town pumps, water from wells, or water carried from streams, rivers, or lakes (whose potability may vary).
3.Indutrial Water

Water (H2O) is essential to most industries. It
is used for a variety of purposes, such as cleaning or dissolving substances.
The amount of water a country needs for industrial purposes varies widely and
is low in mainly rural economies. Most of the water used by industry is not
consumed and can be returned to the water supply. However, it has usually been degraded to some
extent by the processes with which it has been in contact. Environmental
legislation provides for treatment of this wastewater so it can be safely
re-used by the population.

Some industrial wastewater contains hazardous
material such as heavy metals or acids. This needs treatment before entering the
water supply. Often, this will be done on site and may
involve filtration or neutralization. In recent years, industries and their
products have been rated according to the amount of water they use. Water is,
increasingly, a precious resource and industries need to conserve it wherever
possible.

Industries use water in many different ways. It
could be a raw material, as in the food industry or pharmaceutical
manufacturing. Water is said to be the universal solvent, so it is used for
dissolving and diluting, and it also has a high specific heat capacity, so is useful as a coolant for its
ability to absorb the waste heat that is produced by many industrial processes.
Around half of all industrial water withdrawals are used for cooling purposes.
It can also be heated to form steam, which can generate electricity, and so can
be used as a local source of power. In industries making products intended for
human consumption, such as pharmaceuticals or cosmetics, the grade of water
used is important, with various levels of purification being required to remove
toxins and bacteria. Water is also used to transport products and for general
sanitation within an industrial plant.

According to the United Nations World Water Development Report, industry
accounts for 22% of all global water withdrawals. This varies from 59% in
high-income countries, to 8% in low-income countries. This is not as much as is
used by agriculture, which accounts for about 50% of freshwater use.
Agriculture consumes water, mainly in irrigation, returning little of it to the
supply. Industry, however, tends to consume far less of its water withdrawals.
Industry tends to use mainly freshwater, as saltwater is unsuitable for most
applications because it corrodes the metal parts used in machinery. By 2025,
industry will probably account for 24% of global freshwater withdrawals.
Although much industrial water is available for reuse, it is usually degraded
by the processes it has been involved in, and this type of wastewater will
require treatment before its return to the water supply.
The electric power production industry,
comprising hydroelectric, nuclear, and coal and oil-fired power stations,
account for 50% to 70% of industrial water use. Paper and pulp production,
chemicals, mining and metal processing, and petroleum refining all use
substantial amounts of water in their operations. The amount of water used in
producing various goods and services is called water intensity. Manufacture of
a pound of paper takes about 3,000 gallons (11,400 liters) of water, while
producing one car takes, on average, about 65,000 gallons (246,000 liters). A
pound of aluminum takes about 200,000 gallons (757,000 liters) of water to produce
and a hamburger around 1,300 gallons (4,999 liters).
Physical Properties Of Water Sample pH

In
chemistry, pH (/piːˈeɪtʃ/) is a scale used to specify how acidic or basic a
water-based solution is. Acidic solutions have a lower pH, while basic
solutions have a higher pH. At room temperature (25°C or 77°F), pure water is
neither acidic nor basic and has a pH of 7.

The pH scale is logarithmic and inversely indicates the concentration of hydrogen ions in the solution (a lower pH indicates a higher concentration of hydrogen ions). This is because the formula used to calculate pH approximates the negative of the base 10 logarithm of the molar concentration[a] of hydrogen ions in the solution. More precisely, pH is the negative of the base 10 logarithm of the activity of the hydrogen ion.

At 25 °C, solutions with a pH less than 7 are acidic and solutions with a pH greater than 7 are basic. The neutral value of the pH depends on the temperature, being lower than 7 if the temperature increases. The pH value can be less than 0 for very strong acids, or greater than 14 for very strong bases.
The
pH scale is traceable to a set of standard solutions whose pH is established by
international agreement.[3] Primary pH standard values are determined using a
concentration cell with transference, by measuring the potential difference
between a hydrogen electrode and a standard electrode such as the silver
chloride electrode. The pH of aqueous solutions can be measured with a glass
electrode and a pH meter, or a color-changing indicator. Measurements of pH are
important in chemistry, agronomy, medicine, water treatment, and many other
applications.
Pure
water is neutral. When an acid is dissolved in water, the pH will be less than
7 (25 °C). When a base, or alkali, is dissolved in water, the pH will be
greater than 7. A solution of a strong acid, such as hydrochloric acid, at
concentration 1 mol dm−3 has a pH of 0. A solution of a strong alkali, such as
sodium hydroxide, at concentration 1 mol dm−3, has a pH of 14. Thus, measured
pH values will lie mostly in the range 0 to 14, though negative pH values and
values above 14 are entirely possible. Since pH is a logarithmic scale, a
difference of one pH unit is equivalent to a tenfold difference in hydrogen ion
concentration.
1.Temperature

A temperature expresses hot and cold, as measured with a thermometer. In physics, hotness is a body's ability to impart energy as heat to another body that is colder.
Thermometers are calibrated in various temperature scales that historically have used various reference
points and thermometric substances for definition. The most common scales are
the Celsius
scale
(formerly called centigrade), denoted °C, the Fahrenheit scale (denoted °F), and the Kelvin scale (denoted K), the latter of which is
predominantly used for scientific purposes by conventions of the International System of Units (SI).
When a body has no macroscopic chemical reactions
or flows of matter or energy, it is said to be in its own internal state of thermodynamic equilibrium. Its temperature is uniform
in space and unchanging in time.
The lowest theoretical temperature is absolute zero, at which no more thermal energy can be
extracted from a body. Experimentally, it can only be approached very closely,
but not reached, which is recognized in the third law of thermodynamics.
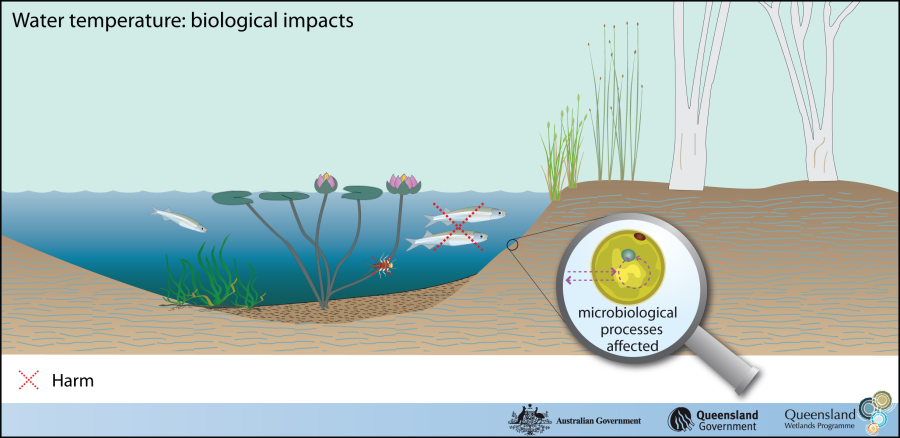
Temperature is important in all fields of natural science, including physics, chemistry, Earth
science, medicine, and biology, as well as most aspects of
daily life.
|
Common symbols |
T |
|
Other units |
|
|
yes |
|
2.Conductivity
Conductivity (or specific conductance) of an electrolyte solution is a measure of its ability to conduct electricity. The SI unit of conductivity is Siemens per meter (S/m).
Conductivity measurements are used routinely in
many industrial and environmental applications as a fast, inexpensive and
reliable way of measuring the ionic content in a solution. For example, the measurement of product
conductivity is a typical way to monitor and continuously trend the performance
of water
purification
systems.
In many cases, conductivity is linked directly
to the total dissolved solids (T.D.S.). High quality deionized water has a
conductivity of about 5.5 μS/m at 25 °C, typical drinking water in the range of
5–50 mS/m, while sea water about 5 S/m[2] (or 5,000,000 μS/m). Conductivity is
traditionally determined by connecting the electrolyte in a Wheatstone bridge. Dilute solutions follow Kohlrausch's Laws of concentration dependence and
additivity of ionic contributions. Lars
Onsager gave
a theoretical explanation of Kohlrausch's law by extending Debye–Hückel theory.
3.Chemical Properties Of Water Sample
Just like animals and humans
living on land, animals that live in water need oxygen to survive. Oxygen from
the atmosphere dissolves in river and lake water, and it is this oxygen that
fish and other aquatic animals use to breathe. When water in creeks and rivers
pours over rocks, oxygen can enter into the water. The picture below shows
rapids in a glacial stream from Ellesmere Island.


Oxygen levels depend on
whether water is flowing or not, whether there are rocks or other obstacles for
water to flow over, how many plants are growing in the water, and the
temperature of the water. There is more oxygen in cold, flowing water with many
obstacles and a moderate amount of plants. Plants take up carbon dioxide and
release oxygen, but if there are too many plants all of the oxygen will be used
up when bacteria decompose them after they die. Oxygen levels are higher in
very cold water compared to very warm water. This might make us think that
water in the winter has lots of oxygen but this is actually not true. During
the winter, ice covers lakes and rivers and very little oxygen enters the water
from the atmosphere– the lake is effectively sealed up. Oxygen in lakes changes
with depth. In deep lakes that do not get very much wind, oxygen levels go down
as we get deeper. In all lakes, oxygen is generally low right at the bottom
where water meets the lake sediment or mud. This is because there are many
bacteria and animals that live and breathe in the sediment. These bacteria and
animals decompose dead material that sinks to the bottom and use up oxygen. In
some lakes and ponds that have very low oxygen, we install aerators to keep
oxygen levels high. This is quite common in lakes that are stocked with fish
and in lakes that receive sewage inputs.
4.Biological Oxygen Demand
(BOD) and Water
Biochemical oxygen demand (BOD) represents the
amount of oxygen consumed by bacteria and other microorganisms while they
decompose organic matter under aerobic (oxygen is present) conditions at a
specified temperature.
When you look at water in a lake the one thing
you don't see is oxygen. In a way, we think that water is the opposite of air,
but the common lake or stream does contain small amounts of oxygen, in the form
of dissolved oxygen. Although the amount of dissolved oxygen is
small, up to about ten molecules of oxygen per million of water, it is a
crucial component of natural water bodies; the presence of a sufficient
concentration of dissolved oxygen is critical to maintaining the aquatic life
and aesthetic quality of streams and lakes.
The presence of a sufficient concentration of
dissolved oxygen is critical to maintaining the aquatic life and aesthetic
quality of streams and lakes. Determining how organic matter affects the concentration
of dissolved oxygen (DO) in a stream or lake is integral to water- quality
management. The decay of organic matter in water is measured as biochemical or
chemical oxygen demand. Oxygen demand is a measure of the amount of
oxidizable substances in a water sample that can lower DO concentrations.
Certain environmental stresses (hot summer
temperatures) and other human-induced factors (introduction of excess fertilizers to a water body) can lessen the amount of
dissolved oxygen in a water body, resulting in stresses on the local aquatic
life. One water analysis that is utilized in order to better understand the
effect of bacteria and other microorganisms on the amount of oxygen they
consume as they decompose organic matter under aerobic (oxygen is present) is
the measure of biochemical oxygen demand (BOD).
Determining how organic matter affects the
concentration of dissolved oxygen in a stream or lake is integral to
water-quality management. BOD is a measure of the amount of oxygen required to
remove waste organic matter from water in the process of decomposition by
aerobic bacteria (those bacteria that live only in an environment containing
oxygen). The waste organic matter is stabilized or made unobjectionable through
its decomposition by living bacterial organisms which need oxygen to do their
work. BOD is used, often in wastewater-treatment plants, as an index of the
degree of organic pollution in water.
5.Chemical
Oxygen Demand (COD)
Chemical
Oxygen Demand (COD) is a second method of estimating how much oxygen would be
depleted from a body of receiving water as a result of bacterial action. While
the BOD test is performed by using a population of bacteria and
other microorganisms to attempt to duplicate what would happen in a
natural stream over a period of five days, the COD test uses a strong chemical oxidizing agent (potassium dichromate or potassium permanganate) to chemically oxidize the
organic material in the sample of wastewater under conditions of heat and
strong acid. The COD test has the advantage of not being subject to
interference from toxic materials, as well as requiring only two or three hours
for test completion, as opposed to five days for the BOD test. It has the
disadvantage of being completely artificial, but is nevertheless considered to
yield a result that may be used as the basis upon which to calculate a
reasonably accurate and reproducible estimate of the oxygen-demanding
properties of a wastewater. The COD test is often used in conjunction with the
BOD test to estimate the amount of nonbiodegradable organic material in a
wastewater. In the case of biodegradable organics, the COD is normally in the
range of 1.3 to 1.5 times the BOD. When the result of a COD test is more than
twice that of the BOD test, there is good reason to suspect that a significant
portion of the organic material in the sample is not biodegradable by ordinary
microorganisms. As a side note, it is important to be aware that the sample
vial resulting from a COD test can contain leachable mercury above regulatory
limits. If such is the case, the sample must be managed as a toxic hazardous waste









No comments